What is Heat?
-
- Energy is transferred between substances as heat. From a microscopic viewpoint, energy transferred as heat tends to move from an object at higher temperature to an object at lower temperature. This is similar to the mechanical behavior of objects moving from a higher gravitational potential energy to a lower gravitational potential energy. Just as a pencil will drop from your desk to the floor but will not jump from the floor to your desk, so energy will travel spontaneously from an object at higher temperature to one at lower temperature and not the other way around.
- The direction in which energy travels as heat can be explained at the atomic level.
- The transfer of energy as heat alters an object’s temperature.
When there is no temperature difference between a substance and its surroundings, no net energy is transferred as heat. Energy transfer depends on the difference of the temperatures of the two objects. The greater the temperature difference is between two objects, the greater the rate of energy transfer between them as heat (other factors being the same).
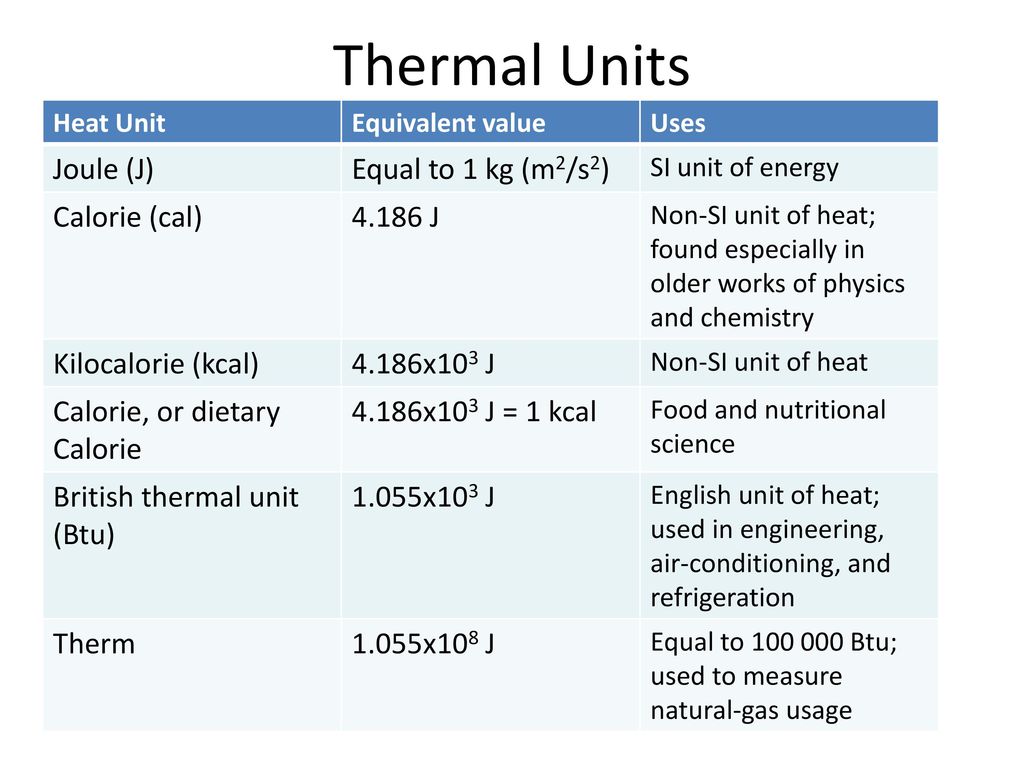
Units of Heat energy:
Temperature and Thermal Equilibrium:
- Adding or removing energy usually changes temperature
Consider what happens when you use an electric range to cook food. By turning the dial that controls the electric current delivered to the heating element, you can adjust the element’s temperature. As the current is increased, the temperature of the element increases. Similarly, as the current is reduced, the temperature of the element decreases. In general, energy must be either added to or removed from a substance to change its temperature. Temperature is proportional to the kinetic energy of of atoms and molecules
- Temperature is meaningful only when it is stable
Thermal equilibrium is the basis for measuring temperature with thermometers. By placing a thermometer in contact with an object and waiting until the column of liquid in the thermometer stops rising or falling, you can find the temperature of the object. The reason is that the thermometer is in thermal equilibrium with the object
- Matter expands as its temperature increases
THERMAL EXPANSION:
Increasing the temperature of a gas at constant pressure causes the volume of the gas to increase. This increase occurs not only for gases but also for liquids and solids. In general, if the temperature of a substance increases, so does its volume. This phenomenon is known as thermal expansion.
THERMAL CONDUCTION:
The rate of thermal conduction depends on the properties of the substance being heated. A metal ice tray and a cardboard package of frozen food removed from the freezer are at the same temperature. However, the metal tray feels colder than the package because metal conducts energy more easily and more rapidly than cardboard does. Substances that rapidly transfer energy as heat are called thermal conductors. Substances that slowly transfer energy as heat are called thermal insulators.
In general, metals are good thermal conductors. Materials such as asbestos, cork, ceramic, cardboard, and fiberglass are poor thermal conductors (and therefore good thermal insulators).
CONVECTION :
Convection involves the movement of cold and hot matter, such as hot air rising upward over a flame. This mechanism does not involve heat alone. Instead, it uses the combined effects of pressure differences, conduction, and buoyancy. In the case of air over a flame, the air is heated through particle collisions (conduction), causing it to expand and its density to decrease. The warm air is then displaced by denser, colder air. Thus, the flame heats the air faster than by conduction alone.
- Such energy transfer occurs when a fluid, such as air or water, comes in contact with an object whose temperature is higher than that of the fluid. The temperature of the part of the fluid that is in contact with the hot object increases, and (in most cases) that fluid expands and thus becomes less dense. Because this expanded fluid is now lighter than the surrounding cooler fluid, buoyant forces cause it to rise. Some of the surrounding cooler fluid then flows so as to take the place of the rising warmer fluid, and the process can then continue.
- Convection is part of many natural processes. Atmospheric convection plays a fundamental role in determining global climate patterns and daily weather variations. Glider pilots and birds alike seek rising thermals (convection currents of warm air) that keep them aloft. Huge energy transfers take place within the oceans by the same process. Finally, energy is transported to the surface of the Sun from the nuclear furnace at its core by enormous cells of convection, in which hot gas rises to the surface along the cell core and cooler gas around the core descends below the surface.
RADIATION:
The other principal energy transfer mechanism is electromagnetic radiation. Unlike convection, energy in this form does not involve the transfer of matter. Instead, objects reduce their internal energy by giving off electromagnetic radiation of particular wavelengths or are heated by electromagnetic radiation like a car is heated by the absorption of sunlight.
SPECIFIC HEAT CAPACITY:
The specific heat of a substance is defined as the energy required to change the temperature of 1 kg of that substance by 1°C.


